At this moment, blockade
of specific growth factor receptors, intracellular targets, and
tyrosine kinase signaling has increased the efficacy of classic chemotherapy
in several cancer types. However, despite this success
a lot of patients do not benefit of the addition of these therapies.
To select patients up front and follow up treatment response,
new tracers and imaging modalities that represent changes in
intra- and extracellular tumor targets, antigens located in the
extracellular matrix or at the blood vessels of tumors during therapy
might support treatment follow-up. As indicated, growth
factor receptors present on the membrane of tumor cells, such as
HER2, EGFR, etc., are suitable candidates for this. Another
approach is to use a downstream product whose transcription is
increased in MDR and by other oncogenic processes. As indicated,
VEGF is such a target that could serve as a specific readout
modality for MDR and the response of new targeted therapies.
Besides intact mAb molecules such as trastuzumab and bevacizumab
(molecular weight, 150 kDa), mAb fragments and engineered
variants are also used, like F(ab)2, F(ab), Fab, single chain
Fv (scFv), and the covalent dimers scFv2, diabodies, and minibodies
(molecular weights ranging from 25 to 100 kDa), as well
as several types of protein therapeutics based on nontraditional
scaffolds, like, for example, domain antibodies, affibodies, nanobodies,
and anticalins could be used for this purpose (85). During
the development of these tracers, one of the main goals should
be to observe whether baseline values and/or changes during
therapy correspond with patient outcome and ultimately patient
survival. All this will lead to more patient-tailored therapy.
Methods Mol Biol. 2010;596:15-31.
Multidrug resistance in oncology and beyond: from imaging of drug efflux pumps to cellular drug targets.
Nagengast WB, Munnink TH, Dijkers EC, Hospers GA, Brouwers AH, Schröder CP, Hooge ML, de Vries EG.
Tuesday, December 8, 2009
Subscribe to:
Post Comments (Atom)
Signal Transduction Pathways
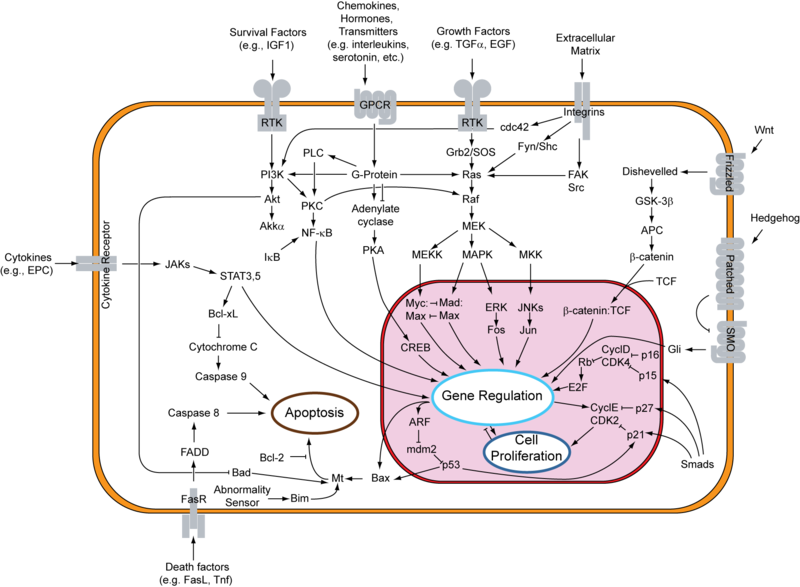
RTK, GPCR, GF, Cytokine, Wnt, Death Factors & Shh pathways
1 comment:
The official NIH definition of a biomarker is: "A characteristic that is objectively measured and evaluated as an indicator of normal biologic processes, pathogenic processes, or pharmacologic responses to a therapeutic intervention." A biomarker has to be reliable, measurable, specific, and predicative.
Post a Comment