 |
| Figure 1: Illustration of HER2 appearance in cancer cell |
In addition to previous reports on the various drugs that are commonly given for the treatment of breast cancer. However, it is important to note that at the molecular level there are different types of breast cancer. One of the most aggressive form is known as HER2 positive type. As the name suggests HER2 over expressing types are considered to be extremely aggressive in nature. It is also considered as an important biomarker in nearly 30% of the breast cancer conditions. Further, 1 in 5 cancer patients shows up-regulation of HER2
protein. Hence, HER2 targeted therapy has gained momentum in last 1 to 2 decades.
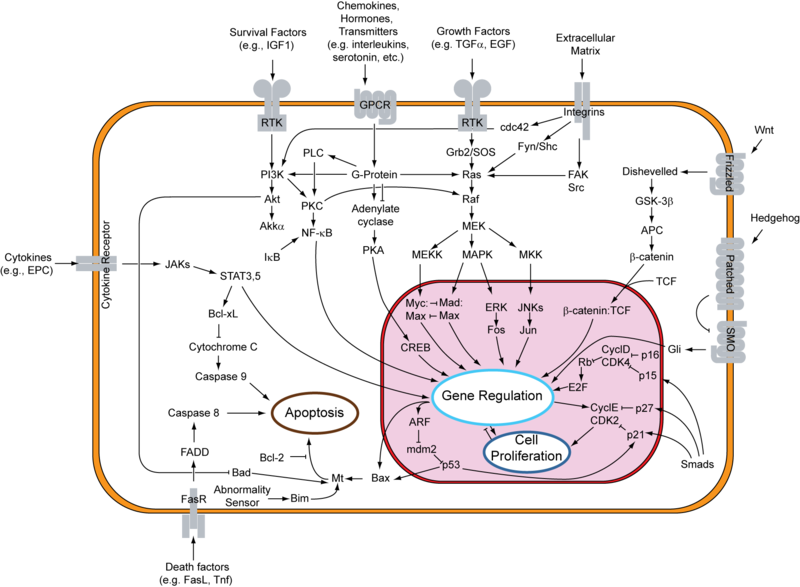
HER2 is a 68kDa protein that helps in the cell growth and development by activation of cascade of cell proliferation pathways associated to RAS, MAP kinases, m-TOR and others as shown in figure 2. HER2 is a mostly expressed as transmembrane protein that makes this as attractive drug target.
HER2 is a 68kDa protein that helps in the cell growth and development by activation of cascade of cell proliferation pathways associated to RAS, MAP kinases, m-TOR and others as shown in figure 2. HER2 is a mostly expressed as transmembrane protein that makes this as attractive drug target.
Figure 2 shows that there are two ways to inhibit HER2 receptor. For e.g extracellular domain as the attacking point for monoclonal antibodies and intracellular domain for small molecule inhibitors.
 |
| Figure 2: Mechanism of action of HER2 receptor and the respective inhibitors to exhibit anti-cancer effect. |
The figure shows that MEK, PKC and IKK inhibitors may aid in the HER2 inhibitors. There has been large amount of efforts been made to develop newer drugs that would target HER2 protein. There are large number of clinical trials that are been conducted for HER2 targeted therapy as shown in table below along with their status.
Table 1: highlights the list drugs that are commonly used for HER2 positive breast cancer patients. It also shows the list of clinical trials under recruiting status by clinicaltrials.gov.in
| HER2 Targeted Drugs | Type | Number of Clinical Trial Studies Under Recruiting Status) |
|---|---|---|
| Trastuzumab (Herceptin) | Monoclonal Antibody | 121 (given in table 2) |
| Pertuzumab (Perjeta) | Monoclonal Antibody | 12 (data download here) |
| Lapatinib (Tykerb) | Small Molecule | 12 (data download here) |
| Ado-trastuzumab emtansine (Kadcyla, also known as TDM-1) | Monoclonal antibody conjugated to cytoxic molecule | 7 (data download here) |
All these drugs are either used in combination of each other or in combination of other drugs that can In order to help the community who are suffering from the HER2 positive breast cancer it will be essential for them to know the clinical trials under recruitment process at National Cancer Institute (NCI).
Table 2: List of clinical trial for HER2 positive breast cancer under treatment where the patients are been recruited for the treatment.
| S. No. | Title | Interventions | Identification Number |
|---|---|---|---|
| 1 | Carboplatin+Nab-paclitaxel, Plus Trastuzumab (HER2+) or Bevacizumab (HER2-) in the Neoadjuvant Setting | Drug: Carboplatin|Drug: paclitaxel albumin-stabilized nanoparticle formulation|Drug: bevacizumab|Drug: trastuzumab|Procedure: magnetic resonance imaging|Procedure: therapeutic conventional surgery | NCT00618657 |
| 2 | An Observational Study of Pregnancy And Pregnancy Outcomes in Women With Breast Cancer Treated With Herceptin, Perjeta In Combination With Herceptin, or Kadcyla During Pregnancy or Within 7 Months Prior To Conception (MotHER) | Biological: Trastuzumab|Biological: HERMyl 1401O Trastuzumab|Drug: Paclitaxel|Drug: Docetaxel | NCT00833963 |
| 3 | Side Effects Involving the Heart in Women With Breast Cancer Receiving Doxorubicin and Trastuzumab | Drug: Afatinib|Drug: Trastuzumab|Drug: Paclitaxel|Drug: Epirubicin|Drug: Cyclophosphamide | NCT00875238 |
| 4 | Assessment of Cardiotoxicity by Cardiac Magnetic Resonance (CMR) in Breast Cancer Patients Receiving Trastuzumab | Procedure: Cardiac MRI|Biological: Biomarker Testing | NCT01022086 |
| 5 | Tolerability of the Combination of Lapatinib and Trastuzumab in Adults Age 60 or Older With HER2 Positive Locally Advanced or Metastatic Breast Cancer | Drug: Lapatinib|Drug: Trastuzumab|Other: laboratory biomarker analysis|Other: pharmacological study | NCT01273610 |
| 6 | Study of BKM120 or BYL719 and Capecitabine in Patients With Metastatic Breast Cancer | Drug: Tesevatinib in combination with Trastuzumab | NCT01300962 |
| 7 | A Study Of Everolimus, Trastuzumab And Vinorelbine In HER2-Positive Breast Cancer Brain Metastases | Drug: Everolimus|Drug: Vinorelbine|Drug: Trastuzumab | NCT01305941 |
| 8 | Intrathecal Trastuzumab for Leptomeningeal Metastases in HER2+ Breast Cancer | Radiation: Trastuzumab | NCT01325207 |
| 9 | Intrathecal Trastuzumab Administration in Metastatic Breast Cancer Patients Developing Carcinomatous Meningitis | Drug: paclitaxel | NCT01373710 |
| 10 | A Study of Pertuzumab in Combination With Trastuzumab Plus an Aromatase Inhibitor in Patients With Hormone Receptor-Positive, Metastatic HER2-positive Breast Cancer | Drug: capecitabine [Xeloda]|Drug: trastuzumab [Herceptin] | NCT01491737 |
| 11 | Efficacy Study of Herceptin to Treat HER2-negative CTC Breast Cancer | Drug: trastuzumab | NCT01548677 |
| 12 | HER2 Imaging Study to Identify HER2 Positive Metastatic Breast Cancer Patient Unlikely to Benefit From T-DM1 | Drug: Trastuzumab|Drug: Gemcitabine | NCT01565200 |
| 13 | Combination Immunotherapy With Herceptin and the HER2 Vaccine NeuVax | Drug: pertuzumab|Drug: Trastuzumab|Drug: Capecitabine|Drug: Paclitaxel|Drug: Vinorelbine|Drug: Docetaxel|Drug: Exemestane|Drug: Letrozole|Drug: Anastrozole|Drug: Fulvestrant|Drug: Tamoxifen | NCT01570036 |
| 14 | Elderly Metastatic Breast Cancer: Pertuzumab-Herceptin vs Pertuzumab-Herceptin-Metronomic Chemotherapy, Followed by T-DM1 | Procedure: PRO Onc Assay and Treatment|Drug: Trastuzumab|Drug: Pertuzumab | NCT01597414 |
| 15 | Neratinib +/- Fulvestrant in Metastatic HER2 Non-amplified But HER2 Mutant Breast Cancer | Drug: Herceptin|Drug: GM-CSF | NCT01670877 |
| 16 | Imaging for Response Assessment of Neoadjuvant Chemotherapy in Primary Breast Cancer (GALADON) | Drug: Albumin-bound paclitaxel|Drug: Carboplatin|Drug: Herceptin® | NCT01690325 |
| 17 | A Combination Study of Kadcyla (Trastuzumab Emtansine) and Capecitabine in Participants With Human Epidermal Growth Factor Receptor 2 (HER2)-Positive Metastatic Breast Cancer (mBC) or HER2-Positive Locally Advanced/Metastatic Gastric Cancer (LA/mGC) | Drug: Intra-arterial Cerebral Infusion of Trastuzumab | NCT01702558 |
| 18 | A Study of Kadcyla (Trastuzumab Emtansine) in Patients With HER2 Positive Breast Cancer Who Have Received Prior Anti-HER2 And Chemotherapy-based Treatment | Drug: Trastuzumab|Drug: GRN 1005 | NCT01702571 |
| 19 | CharactHer. ICORG 12-09, V3 | Drug: trastuzumab|Drug: vinorelbine | NCT01722890 |
| 20 | Ad/HER2/Neu Dendritic Cell Cancer Vaccine Testing | Drug: Afatinib once daily (OD)|Drug: Vinorelbine Weekly | NCT01730118 |
| 21 | Lapatinib+Vinorelbine vs Vinorelbine HER2 Positive Metastatic Breast Cancer Progressed After Lapatinib/Trastuzumab | Drug: Epirubicin|Drug: Docetaxel|Drug: Trastuzumab|Drug: Carboplatin | NCT01730677 |
| 22 | Pertuzumab, Trastuzumab, and Paclitaxel Albumin-Stabilized Nanoparticle Formulation in Treating Patients With HER2-Positive Advanced Breast Cancer | Biological: pertuzumab|Biological: trastuzumab|Drug: paclitaxel albumin-stabilized nanoparticle formulation|Other: laboratory biomarker analysis | NCT01730833 |
| 23 | Paclitaxel and Cyclophosphamide With or Without Trastuzumab Before Surgery in Treating Patients With Previously Untreated Stage I-III Breast Cancer | Drug: paclitaxel|Drug: cyclophosphamide|Biological: trastuzumab|Procedure: therapeutic conventional surgery|Radiation: radiation therapy|Drug: doxorubicin hydrochloride|Other: laboratory biomarker analysis | NCT01750073 |
| 24 | HELENA Study: An Observational Study of Perjeta (Pertuzumab) in First-Line Treatment in Patients With Her2-Positive Advanced Breast Cancer After Adjuvant Herceptin Therapy | Drug: Docetaxel|Drug: Carboplatin|Drug: Herceptin|Drug: Vinorelbine | NCT01777958 |
| 25 | Effect of Trastuzumab on Disease Free Survival in Early Stage HER2-Negative Breast Cancer Patients With ERBB2 Expressing Disseminated Tumor Cells | Drug: non-pegylated liposomal doxorubicin|Drug: Carboplatin|Drug: Paclitaxel|Drug: Epirubicin|Drug: Cyclophosphamide|Drug: Pertuzumab|Drug: Trastuzumab|Drug: Ferric carboxymaltose | NCT01779050 |
| 26 | Phase 1b/2 Trial Using Lapatinib, Everolimus and Capecitabine for Treatment of HER-2 Positive Breast Cancer With CNS Metastasis | Drug: nab-paclitaxel | NCT01783756 |
| 27 | Pre Operative Trastuzumab in Operable Breast Cancer | Drug: docetaxel+lapatinib|Drug: docetaxel + trastuzumab|Drug: docetaxel + trastuzumab + lapatinib | NCT01785420 |
| 28 | Paclitaxel + Trastuzumab + Pertuzumab as Pre-Op for Inflammatory BrCa | Drug: Lapatinib and Capecitabine and Vinorelbine | NCT01796197 |
| 29 | Imaging With 111 Indium (111In)-Pertuzumab (PmAb) to Predict Response to Trastuzumab (TmAb) in Human Epidermal Growth Factor-2 (HER2) Positive Metastatic Breast Cancer (MBC) or Locally Advanced Breast Cancer (LABC) | Drug: Palbociclib|Drug: Trastuzumab|Drug: Letrozole | NCT01805908 |
| 30 | Ado-Trastuzumab Emtansine in Treating Patients With HER2-Positive Metastatic or Locally Advanced Breast Cancer That Cannot Be Removed by Surgery | Drug: docetaxel|Drug: pertuzumab|Drug: trastuzumab emtansine | NCT01816035 |
| 31 | A Prospective, Randomized Multicenter, Open-label Comparison of Preoperative Combination of Trastuzumab and Pertuzumab With or Without Concurrent Taxane Chemotherapy Given for Twelve Weeks in Patients With Operable HER2+/HR- Breast Cancer Within the ADAPT Protocol | Drug: Lapatinib|Biological: Trastuzumab | NCT01817452 |
| 32 | Phase 2 Study of the Monoclonal Antibody MGAH22 (Margetuximab) in Patients With Relapsed or Refractory Advanced Breast Cancer | Drug: Lapatinib|Biological: Trastuzumab | NCT01828021 |
| 33 | Identification of Early Markers of Response and Resistance to Trastuzumab | Drug: Metoprolol|Drug: Placebo|Drug: Candesartan|Drug: Placebo | NCT01834950 |
| 34 | Study of Propranolol in Newly Diagnosed Breast Cancer Patients Undergoing Neoadjuvant Chemotherapy | Drug: trastuzumab (Herceptin®)|Drug: anastrazole (Arimidex®) | NCT01847001 |
| 35 | NSABP Biospecimen Discovery Project | Drug: BEZ235 + paclitaxel|Drug: BKM120 + paclitaxel|Drug: BEZ235 + paclitaxel + trastuzumab|Drug: BKM120 + paclitaxel + trastuzumab | NCT01850628 |
| 36 | T-DM1 vs Paclitaxel/Trastuzumab for Breast (ATEMPT Trial) | Biological: trastuzumab | NCT01853748 |
| 37 | Phase 2 Trial of Pertuzumab and Trastuzumab With Weekly Paclitaxel and Chemotherapy for HER2 Positive Breast Cancer | Drug: LJM716|Drug: Trastuzumab | NCT01855828 |
| 38 | Capecitabine, Cyclophosphamide, Lapatinib Ditosylate, and Trastuzumab in Treating Patients With HER2-Positive Metastatic Breast Cancer | Drug: capecitabine|Drug: cyclophosphamide|Drug: lapatinib ditosylate|Biological: trastuzumab|Other: laboratory biomarker analysis | NCT01873833 |
| 39 | Defining the HER2 Positive (+) Breast Cancer Kinome Response to Trastuzumab, Pertuzumab, Combination Trastuzumab +Pertuzumab, or Combination Trastuzumab + Lapatinib | Drug: Trastuzumab|Drug: pertuzumab|Drug: lapatinib | NCT01875666 |
| 40 | Efficacy and Safety of Bevacizumab in the Neodjuvant Treatment of Inflammatory Breast Cancer | Drug: Standard chemotherapy|Drug: bevacizumab [Avastin]|Drug: trastuzumab [Herceptin] | NCT01880385 |
| 41 | Phase II Trial to Validate Markers for a Response Evaluation of a Combined Therapy in Patients With HER2+ Breast Cancer | Biological: Margetuximab | NCT01891357 |
| 42 | Chemotherapy Before Surgery and Tissue Sample Collection in Patients With Stage IIA-IIIC Breast Cancer | Drug: Cyclophosphamide|Other: Cytology Specimen Collection Procedure|Drug: Doxorubicin Hydrochloride|Other: Laboratory Biomarker Analysis|Drug: Paclitaxel|Biological: Trastuzumab | NCT01897441 |
| 43 | Cardiac Safety Study in Patients With HER2 + Breast Cancer | Drug: Herceptin|Drug: Taxol|Drug: Fluorouracil|Drug: Cytoxan|Drug: Epirubicin | NCT01904903 |
| 44 | Phase II Study of Eribulin Mesylate, Trastuzumab, and Pertuzumab in Women With Metastatic, Unresectable Locally Advanced, or Locally Recurrent HER2-Positive Breast Cancer | Drug: Pertuzumab, Trastuzumab and Eribulin | NCT01912963 |
| 45 | Pertuzumab and Trastuzumab as Neoadjuvant Treatment in Patients With HER2-Positive Breast Cancer | Drug: fulvestrant 500 mg|Drug: Docetaxel (T) 75 mg/m2 (Taxotere)|Drug: Trastuzumab (H, 8mg/kg|Drug: Pertuzumab (P, 840 mg | NCT01937117 |
| 46 | Trastuzumab Combined With Chemotherapy or Endocrine Therapy to Treat Metastatic Luminal B2 Breast Cancer | Drug: doxorubicine, cyclophosphamide, docetaxel|Drug: doxorubicine, cyclophosphamide, trastuzumab, docetaxel|Drug: trastuzumab, docetaxel, carboplatin | NCT01950182 |
| 47 | IMaging PAtients for Cancer Drug selecTion - Metastatic Breast Cancer | Drug: Standard Therapy|Drug: AMG 386|Drug: AMG 479 (Ganitumab) plus Metformin|Drug: MK-2206 with or without Trastuzumab|Drug: AMG 386 and Trastuzumab|Drug: T-DM1 and Pertuzumab|Drug: Pertuzumab and Trastuzumab|Drug: Ganetespib|Drug: ABT-888|Drug: Neratinib|Drug: PLX3397|Drug: Pembrolizumab | NCT01957332 |
| 48 | Trastuzumab and Pertuzumab or Bevacizumab With Combination Chemotherapy in Treating Patients With Stage II-III Breast Cancer | Biological: trastuzumab|Biological: Pertuzumab|Drug: docetaxel|Drug: carboplatin|Drug: doxorubicin|Drug: cyclophosphamide|Drug: paclitaxel|Other: laboratory biomarker analysis|Other: pharmacogenomic studies|Drug: Bevacizumab | NCT01959490 |
| 49 | Validity of HER2-amplified Circulating Tumor Cells to Select Metastatic Bresat Cancer Considered HER2-negative for Trastuzumab-emtansine (T-DM1) Treatment. | Drug: bevacizumab|Drug: no bevacizumab | NCT01975142 |
| 50 | Phase 1b Study of PD-0332991 in Combination With T-DM1(Trastuzumab-DM1) | Drug: Trastuzumab|Drug: Pertuzumab|Drug: Palbociclib|Drug: Fulvestrant | NCT01976169 |
| 51 | NK Cell Infusions With Trastuzumab for Patients With HER2+ Breast and Gastric Cancer | Drug: Lapatinib|Biological: Trastuzumab | NCT02030561 |
| 52 | A Study of Subcutaneous Herceptin (Trastuzumab) Administered at Home By Single-Use Injection Device in Patients With Early HER2-Positive Breast Cancer (HOMERUS) | Drug: Lapatinib plus trastuzumab | NCT02040935 |
| 53 | Study of Optimizing Neoadjuvant Regimens in Subtypes of Breast Cancer | Drug: Nab-Paclitaxel | NCT02041338 |
| 54 | Ganetespib, Paclitaxel, Trastuzumab and Pertuzumab for Metastatic Human Epidermal Growth Factor Receptor 2 Positive Breast Cancer | Drug: ganetespib|Drug: paclitaxel|Biological: trastuzumab|Biological: pertuzumab | NCT02060253 |
| 55 | 89ZrTrastuzumab Breast Imaging With Positron Emission Tomography | Drug: Epothilone D|Drug: Herceptin | NCT02065609 |
| 56 | Ruxolitinib in Combination With Trastuzumab in Metastatic HER2 Positive Breast Cancer | Drug: Ruxolitinib|Drug: Trastuzumab | NCT02066532 |
| 57 | Neoadjuvant TDM1 With Lapatinib and Abraxane Compared With Trastuzumab Plus Pertuzumab With Paclitaxel | Drug: paclitaxel plus trastuzumab | NCT02073487 |
| 58 | TDM1 With Abraxane and Lapatinib for Metastatic HER2 Positive Breast Cancer | Drug: docetaxel + trastuzumab sc + pertuzumab|Drug: trastuzumab emtansin | NCT02073916 |
| 59 | Strain Imaging in Breast Cancer Patients Receiving Trastuzumab | Drug: BMK120|Drug: Capecitabine|Drug: BYL719|Drug: Trastuzumab|Drug: Lapatinib | NCT02080390 |
| 60 | A Study to Assess the Efficacy and Safety of Enzalutamide With Trastuzumab in Subjects With Human Epidermal Growth Factor Receptor 2 Positive (HER2+), Androgen Receptor Positive (AR+) Metastatic or Locally Advanced Breast Cancer | Drug: enzalutamide|Drug: trastuzumab | NCT02091960 |
| 61 | Cardiotoxicity in Metastatic Her 2 Positive Patients Treated With Trastuzumab ,Pertuzumab and Taxanes | Drug: Paclitaxel and carboplatin|Drug: Paclitaxel|Drug: Epirubicin and Paclitaxel | NCT02101879 |
| 62 | A Phase III Trial Comparing Two Dose-dense, Dose-intensified Approaches (ETC and PM(Cb)) for Neoadjuvant Treatment of Patients With High-risk Early Breast Cancer (GeparOcto) | Drug: Trastuzumab emtansine | NCT02125344 |
| 63 | Anti-PD-1 Monoclonal Antibody in Advanced, Trastuzumab-resistant, HER2-positive Breast Cancer | Drug: HBI-8000|Drug: Trastuzumab|Drug: Paclitaxel | NCT02129556 |
| 64 | Phase I/IIa Trial of Gemcitabine Plus Trastuzumab and Pertuzumab in Previously Treated Metastatic HER2+ Breast Cancer | Drug: BIBW 2992|Drug: trastuzumab|Drug: vinorelbine|Drug: vinorelbine | NCT02139358 |
| 65 | Everolimus, Letrozole and Trastuzumab in HR- and HER2/Neu-positive Patients | Other: 111In-Pertuzumab + SPECT-CT | NCT02152943 |
| 66 | Open-Label Study Evaluating the Safety and Tolerability of LJM716, BYL719 and Trastuzumab in Patients With Metastatic HER2+ Breast Cancer | Drug: Lapatinib|Drug: Trastuzumab | NCT02167854 |
| 67 | Carvedilol for the Prevention of Anthracycline/Anti-HER2 Therapy Associated Cardiotoxicity Among Women With HER2-Positive Breast Cancer Using Myocardial Strain Imaging for Early Risk Stratification | Biological: MGAH22 | NCT02177175 |
| 68 | An Open-Label Randomized Study to Investigate the Tolerability of Subcutaneous (SC) Trastuzumab Administration in Patients With HER2-Positive Early Breast Cancer (eBC) Using Either a Single-Use Injection Device or Manual Administration | Drug: HKI-272 (neratinib)|Drug: HKI-272 (neratinib) | NCT02194166 |
| 69 | Evaluation of Biomarkers Associated With Response to Subsequent Therapies in Subjects With HER2-Positive Metastatic Breast Cancer | Device: PICC|Device: PORT | NCT02213042 |
| 70 | MM-302 Plus Trastuzumab vs. Chemotherapy of Physician's Choice Plus Trastuzumab in HER2-Positive Locally Advanced/Metastatic Breast Cancer Patients | Drug: MM-302|Drug: Gemcitabine|Drug: Capecitabine|Drug: Vinorelbine|Drug: Trastuzumab | NCT02213744 |
| 71 | Neoadjuvant Trastuzumab and Letrozole for Postmenopausal Women | Drug: pertuzumab|Drug: pertuzumab|Drug: trastuzumab [Herceptin]|Drug: trastuzumab [Herceptin]|Drug: vinorelbine | NCT02214004 |
| 72 | Copper Cu 64-DOTA-Trastuzumab PET in Predicting Response to Treatment With Ado-Trastuzumab Emtansine in Patients With Metastatic HER2 Positive Breast Cancer | Radiation: fludeoxyglucose F 18|Procedure: positron emission tomography|Procedure: computed tomography|Biological: trastuzumab|Radiation: copper Cu 64-DOTA-trastuzumab|Procedure: positron emission tomography|Biological: ado-trastuzumab emtansine|Other: laboratory biomarker analysis | NCT02226276 |
| 73 | Phase 2 Study of Standard Chemotherapy With Trastuzumab, Plus or Minus Pertuzumab, for Pre-treated Metastatic Breast Cancer | Drug: Trastuzumab|Drug: Pertuzumab|Drug: Vinorelbine, Paclitaxel, Nab-Paclitaxel , Docetaxel, Capecitabine | NCT02229149 |
| 74 | A Dose-Escalation Study Evaluating the Combination of Trastuzumab Emtansine (T-DM1) With Neratinib in Women With Metastatic HER2-Positive Breast Cancer | Drug: pegylated liposomal doxorubicin | NCT02236000 |
| 75 | Cardiotoxicity Prevention in Breast Cancer Patients Treated With Anthracyclines and/or Trastuzumab | Drug: Neratinib|Drug: Fulvestrant|Drug: Trastuzumab | NCT02236806 |
| 76 | Safety and QoL of Trastuzumab With Lapatinib or Chemiotherapy in MBC and HER2+ Patients Refractory to Anti HER2 Therapies | Drug: INCB007839 300mg BID|Drug: Trastuzumab|Drug: Vinorelbine | NCT02238509 |
| 77 | Gemcitabine, Trastuzumab, and Pertuzumab in the Treatment of Metastatic HER2-Positive Breast Cancer After Prior Trastuzumab/Pertuzumab, or Pertuzumab Based Therapy | Drug: Everolimus|Drug: Placebo | NCT02252887 |
| 78 | Cabozantinib +/- Trastuzumab In Breast Cancer Patients w/ Brain Metastases | Drug: Cabozantinib|Drug: Trastuzumab | NCT02260531 |
| 79 | Can HER2 Targeted 89Zr-trastuzumab PET/CT Identify Unsuspected HER2 Positive Breast Cancer Metastases, Which Are Amenable to HER2 Targeted Therapy? | Radiation: 89Zr-trastuzumab|Device: PET/CT scan | NCT02286843 |
| 80 | Phase II Trial of Combination Immunotherapy With NeuVax and Trastuzumab in High-risk HER2+ Breast Cancer Patients | Biological: NeuVax vaccine|Drug: Trastuzumab|Drug: GM-CSF | NCT02297698 |
| 81 | An Observational Study of Herceptin SC Safety in Breast Cancer | Biological: HER2 CTL vaccine (plus trastuzumab) | NCT02305628 |
| 82 | An Observational Study of Kadcyla Safety in Breast Cancer | Drug: Docetaxel|Drug: pertuzumab [Perjeta]|Drug: trastuzumab [Herceptin] | NCT02305641 |
| 83 | Pembrolizumab and Monoclonal Antibody Therapy in Advanced Cancer | Other: Standard Dose Dense Doxorubucin and Cyclophosphomide|Other: Standard Doxorubucin and Cyclophosphomide|Other: Standard Docetaxel, Carboplatin, and Herceptin|Other: Standard Docetaxel, Doxorubucin and Cyclophosphomide|Other: Standard Docetaxel and Cyclophosphomide | NCT02318901 |
| 84 | T-DM1+Pertuzumab in Pre-OP Early-Stage HER2+ BRCA | Drug: Herceptin|Drug: afatinib|Drug: trastuzumab|Drug: Herceptin|Drug: afatinib|Drug: afatinib | NCT02326974 |
| 85 | Study of ASLAN001 in Combination With Capecitabine in MBC That Has Failed on Prior Trastuzumab | Drug: Herceptin™ (Her)|Drug: Herceptin™ (Her) + chemo | NCT02338245 |
| 86 | Neoadjuvant Phase II Trial in Patients With T1c Operable, HER2-positive Breast Cancer According to TOP2A Status | Drug: Kadcyla (trastuzumab emtansine) | NCT02339532 |
| 87 | Detect V / CHEVENDO (Chemo vs. Endo) | Drug: TAC chemotherapy|Drug: TC chemotherapy|Drug: Dose Dense AC or FEC100 followed by paclitaxel or docetaxel chemotherapy|Drug: TCH chemotherapy|Drug: T + trastuzumab followed by CEF + trastuzumab|Drug: Dose dense AC followed by T + trastuzumab|Drug: Dose dense AC followed by T + trastuzumab + pertuzumab|Drug: PTH followed by dose dense AC of FEC | NCT02344472 |
| 88 | Neoadjuvant Hormonal Therapy Combined With Chemoimmunotherapy (Taxotere, Trastuzumab and Pertuzumab) in Patients With HER2-positive and ER-Positive Breast Cancer | Radiation: radiation therapy|Drug: Capecitabine|Drug: Trastuzumab|Drug: Paclitaxel | NCT02345772 |
| 89 | A Multi-Center Study of Lapatinib in Patients With Trastuzumab-refractory Metastatic Breast Cancer | Drug: capecitabine [Xeloda]|Drug: Taxotere|Drug: Herceptin (HER2-neu positive patients only)|Drug: capecitabine [Xeloda]|Drug: Taxotere | NCT02362958 |
| 90 | Phase Ib Dose-escalation Trial of Taselisib (GDC-0032) in Combination With Anti-HER2 Therapies in Participants With Advanced HER2+ Breast Cancer | Drug: GDC-0941|Drug: Trastuzumab|Drug: trastuzumab-MCC-DM1 | NCT02390427 |
| 91 | Dose-confirmation Study of ASLAN001 Combined With Weekly Paclitaxel and Carboplatin in Advanced Solid Tumours, Followed by a Study in Patients With Stage I-III HER2 Positive Breast Cancer | Drug: Myocet|Drug: Taxotere|Drug: Herceptin | NCT02396108 |
| 92 | A Study Looking the Incidence and Severity of Diarrhea in Patients With Early-Stage HER2+ Breast Cancer Treated With Neratinib and Loperamide | Other: Blood, Echocardiography, and Questionnaire Timepoints for Anthracycline abd Trastuzumab|Other: Blood, Echocardiography and Questionnaire for Trastuzumab|Other: Blood, Echocardiography and Questionnaire for Anthracycline Regimen | NCT02400476 |
| 93 | Phase IIIb Study to Evaluate the Safety and Tolerability of Herceptin SC With Perjeta and Docetaxel in Patients With HER2-positive Advanced Breast Cancer | Drug: Temsirolimus|Drug: Neratinib | NCT02402712 |
| 94 | Preoperative Study With Trastuzumab, Pertuzumab and Letrozole in Patients With Breast Cancer Sensitive to Hormonal Therapy | Drug: bevacizumab|Drug: vinorelbine|Drug: trastuzumab | NCT02411344 |
| 95 | Trastuzumab Emtansine in Treating Older Patients With Human Epidermal Growth Factor Receptor 2-Positive Stage I-III Breast Cancer | Biological: Trastuzumab Emtansine|Other: Questionnaire Administration|Other: Laboratory Biomarker Analysis|Other: Quality-of-Life Assessment | NCT02414646 |
| 96 | Safety and Efficacy of Trastuzumab as Part of Breast Cancer Treatment Regimen | Drug: vinorelbine|Drug: carboplatin|Drug: trastuzumab | NCT02419742 |
| 97 | Physical Activity Intervention on Myocardial Function in Patients With HER2 + Breast Cancer | Drug: lapatinib and capecitabine | NCT02433067 |
| 98 | Breast Cancer Treatment Using Weekly Carboplatin + Paclitaxel With Pertuzumab + Trastuzumab (HER2+) or Bevacizumab (HER2-) in the Neoadjuvant Setting | Drug: trastuzumab|Drug: chemotherapy | NCT02436993 |
| 99 | Cardiac Toxicity in Medical Treatment of Breast Cancer | Other: Cardiac MRI | NCT02440620 |
| 100 | Safety Study of Pertuzumab (In Combination With Trastuzumab and Docetaxel) in Indian Patients With Breast Cancer | Drug: Gemcitabine|Drug: Carboplatin|Drug: Trastuzumab | NCT02445586 |
| 101 | Study of Palbociclib and Trastuzumab With or Without Letrozole in HER2-positive Metastatic Breast Cancer | Drug: G-CSF|Drug: trastuzumab|Drug: vinorelbine|Drug: G-CSF|Drug: saline | NCT02448420 |
| 102 | A Study of Liposomal Doxorubicin + Docetaxel + Trastuzumab + Metformin in Operable and Locally Advanced HER2 Positive Breast Cancer | Drug: Liposomal doxorubicin|Drug: Docetaxel|Drug: Trastuzumab|Drug: Metformin | NCT02488564 |
| 103 | Margetuximab Plus Chemotherapy vs Trastuzumab Plus Chemotherapy in the Treatment of HER2+ Metastatic Breast Cancer | Drug: Trastuzumab, Pertuzumab, Ado-trastuzumab emtansine | NCT02492711 |
| 104 | A Study on Neoadjuvant Therapy for Her-2 Positive Breast Cancer and the Prognosis by Detecting CTCs | Biological: recombinant interleukin-12|Biological: ABI-007/carboplatin/trastuzumab | NCT02510781 |
| 105 | A Randomized, Open-label Phase III Trial to Evaluate the Efficacy and Safety of Pertuzumab Retreatment in Previously Pertuzumab, Trastuzuamb and Chemotherapy Treated Her2-Positive Metastatic Locally Advanced and Metastatic Breast Cancer(Study of Perjeta Re-treatment for Clinical Outcomes) | Drug: Doxorubicin|Drug: cyclophosphamide|Drug: paclitaxel|Drug: docetaxel|Drug: Trastuzumab|Drug: Pertuzumab | NCT02514681 |
| 106 | Pertuzumab With High-Dose Trastuzumab in Human Epidermal Growth Factor Receptor 2 (HER2)-Positive Metastatic Breast Cancer (MBC) With Central Nervous System (CNS) Progression Post-Radiotherapy | Drug: Trastuzumab emtansine | NCT02536339 |
| 107 | Long-term Outcome of HER2-amplified Metastatic Breast Cancer: A Retrospective Analysis | Other: Biopsy | NCT02560311 |
| 108 | T-DM1 and Non-pegylated Liposomal Doxorubicin in HER2-positive Metastatic Breast Cancer | Drug: E7389 | NCT02562378 |
| 109 | Neoadjuvant Response-guided Treatment of HER2 Positive Breast Cancer | Biological: trastuzumab|Drug: capecitabine|Drug: paclitaxel | NCT02568839 |
| 110 | Super-selective Intra-arterial Cerebral Infusion of Trastuzumab for the Treatment of Cerebral Metastases of HER2/Neu Positive Breast Cancer | Drug: Zoledronic acid | NCT02571530 |
| 111 | A Randomized, Double-Blind, Placebo-Controlled Study in Patients With Early-Stage or Locally Advanced HER2-positive Breast Cancer to Evaluate Treatment With Trastuzumab + Pertuzumab + Docetaxel Compared With Trastuzumab + Placebo + Docetaxel | Drug: Epirubicin|Drug: Docetaxel|Drug: Capecitabine|Drug: Trastuzumab | NCT02586025 |
| 112 | Neoadjuvant Nab-PTX and Trastuzumab for ER Negative and HER2 Positive Breast Cancer | Drug: nab-paclitaxel|Drug: Trastuzumab | NCT02598310 |
| 113 | Safety and Pharmacokinetics of Atezolizumab in Combination With Trastuzumab Emtansine or With Trastuzumab and Pertuzumab in Participants With HER2-Positive Breast Cancer | Drug: Doxorubicin|Biological: Trastuzumab|Drug: Cyclophosphamide|Drug: Paclitaxel|Drug: Epirubicin|Drug: Docetaxel|Drug: Carboplatin|Drug: Fluorouracil | NCT02605915 |
| 114 | Study of ONT-380 vs Placebo in Combo w/ Capecitabine & Trastuzumab in Patients w/ Metastatic HER2+ Breast Cancer | Drug: trastuzumab, docetaxel and carboplatin in dose dense regimen | NCT02614794 |
| 115 | Assessment for Long-Term Cardiovascular Impairment Associated With Trastuzumab Cardiotoxicity in HER2-Positive Breast Cancer Survivors | Drug: Lapatinib|Drug: Doxorubicin|Drug: Cyclophosphamide|Drug: Docetaxel|Drug: pegfilgrastim|Drug: filgrastim|Drug: dexamethasone|Drug: trastuzumab | NCT02615054 |
| 116 | PErsonalized TREatment of High-risk MAmmary Cancer - the PETREMAC Trial | Drug: Trastuzumab | NCT02624973 |
| 117 | Adjuvant Trastuzumab, Pertuzumab Plus Docetaxel in the Treatment of Early HER2-positive Breast Cancer | Drug: Pertuzumab|Drug: Trastuzumab | NCT02625441 |
| 118 | Pertuzumab in First Line Treatment of HER2-positive Metastatic Breast Cancer Patients | Drug: lapatinib|Drug: carboplatin|Drug: trastuzumab|Drug: paclitaxel | NCT02642458 |
| 119 | microRNA of Human Epidermal Growth Factor Receptor 2 (HER2)Positive Patient Treated With Herceptin | Other: Blood test | NCT02656589 |
| 120 | An Open-Label, Phase Ib/II Clinical Trial Of Cdk 4/6 Inhibitor, Ribociclib (Lee011), In Combination With Trastuzumab Or T-Dm1 For Advanced/Metastatic Her2-Positive Breast Cancer. | Drug: Durvalumab|Drug: Trastuzumab | NCT02657343 |
| 121 | Trial of Neoadjuvant Trastuzumab Emtansine in Patients With HER2-Equivocal Breast Cancer | Drug: Trastuzumab emtansine | NCT02725541 |
No comments:
Post a Comment